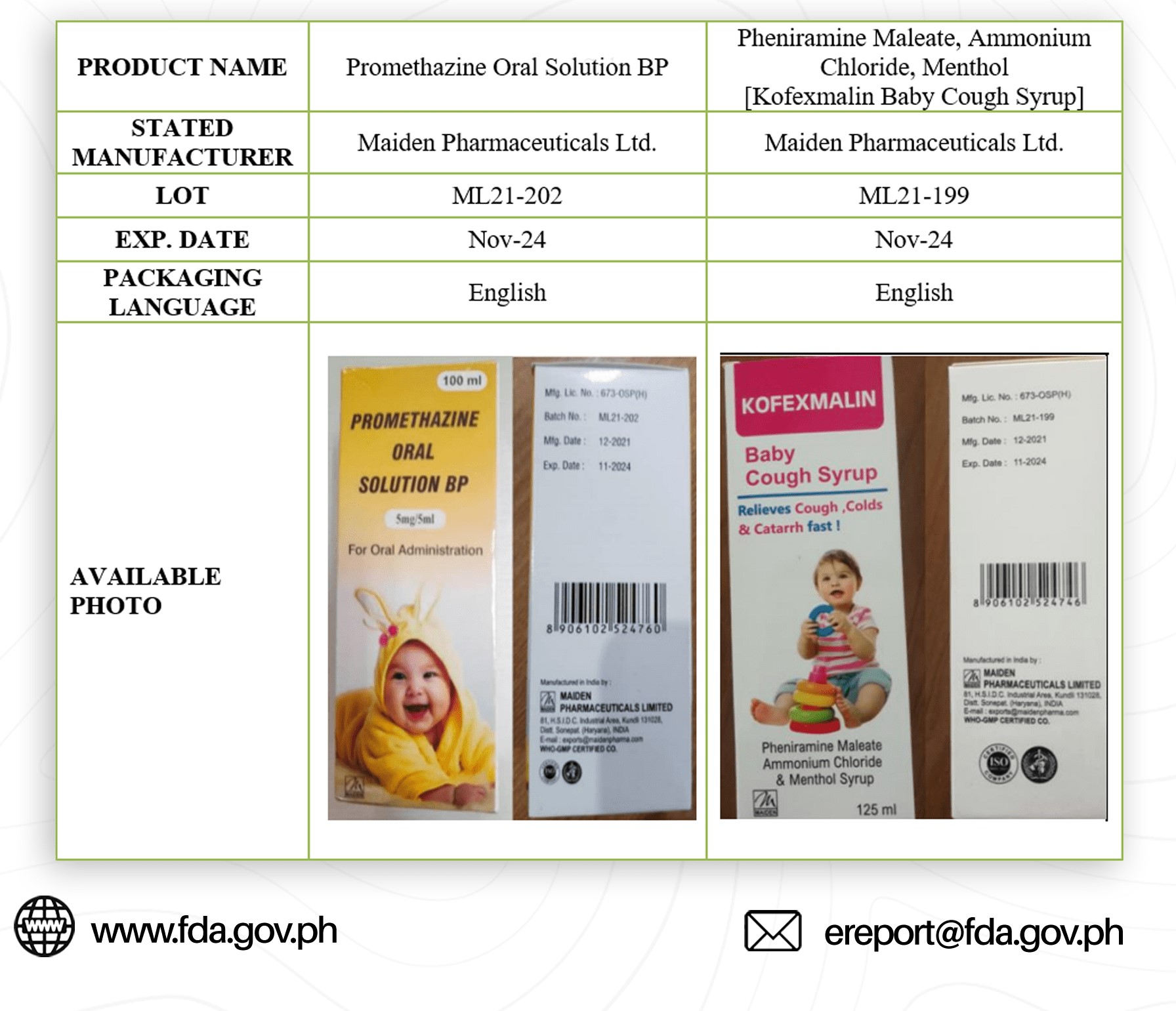
The Food and Drug Administration (FDA) has issued a warning to the public against purchasing and consuming four India-made brands of cough and cold syrup medicines for kids.
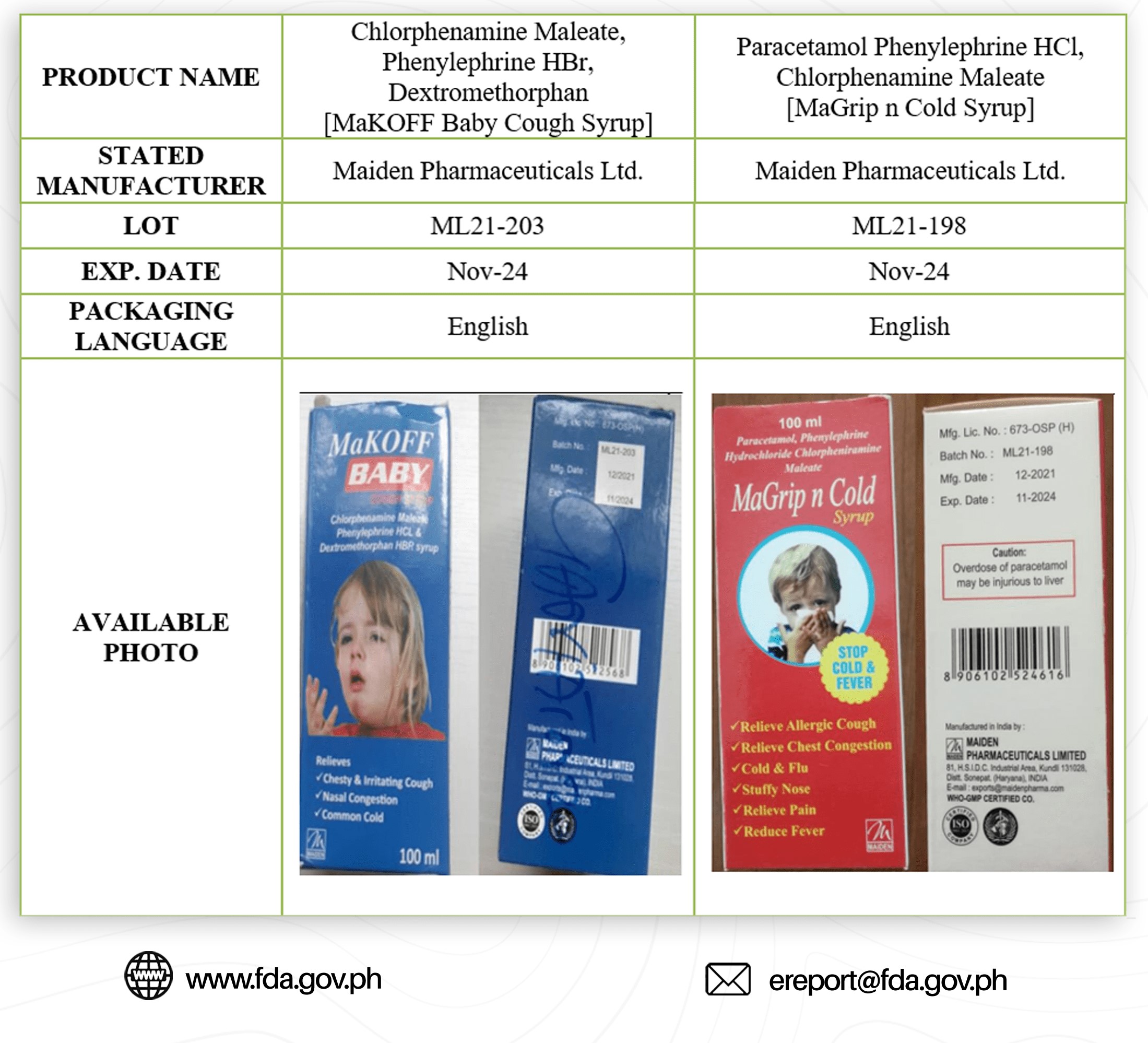
In an advisory issued on Oct. 21, FDA identified the brands as Promethazine Oral Solution BP; Pheniramine Maleate, Ammonium Chloride, Menthol; Chlorphenamine Maleate, Phenylephrine HBr, Dextromethorphan; and Paracetamol Phenylephrine HCl, Chlorphenamine Maleate.
It said that these medicine products has contaminants like Diethylene Glycol and Ethylene Glycol which are considered “toxic” to the human body.
FDA said that once consumed, it may cause abdominal pain, vomiting, diarrhea, inability to pass urine, headache, altered mental state, and acute kidney injury that may lead to death.
“To date, the stated manufacturer has not provided guarantees to WHO [World Health Organization] on the safety and quality of these products,” it said.
“All batches of these products should be considered unsafe until they can be analyzed by the relevant National Regulatory Authorities,” FDA added.
It reiterated that the substandard drug products reported are not FDA-registered.
The agency requested the Local Government Units (LGU) and Law Enforcement Agencies (LEAs) to monitor and ensure that drug products are not sold nor distributed to patients in their localities or areas of jurisdiction.